The latest edition of the Cosmetics Toiletries and Fragrance Association (CTFA) Dictionary lists more than 10,000 raw materials. Every year hundreds of new ingredients are added to the list of those that have been used for centuries. Some materials used today can be traced to 11,000 B.C.E. in the animal drawings from the caves of Altimira.
History
The appearance of skin care formulation dates to around 3000 B.C.E. in ancient Egypt. Most concoctions were prepared from natural materials. Cleopatra is said to have bathed in donkeys’ milk to keep her skin smooth and supple. One naturally occurring material used by the ancients was red ochre, or iron oxide. Lumps of red ore were formed when iron oxidized or rusted. The red iron oxide was found in burial tombs in ceremonial lip tints and rouge preparations. It was also used to draw the ancient cave pictures of animals, as seen in Altimira, and is still used in many makeup formulations
today. Eye paints have also been found at ancient gravesites. These paints consisted primarily of a copper-based green ore called malachite that was mined from nearby quarries. Animal fat was combined with fragrant substances such as frankincense and myrrh to produce early skin ointments. More sophisticated creams and lotions were fine tuned through trial and error and passed down over many generations.
Emulsions
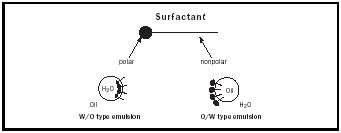
The majority of creams and lotions are emulsions. An emulsion can be defined simply as two immiscible fluids in which one liquid is dispersed as fine droplets in the other. Homogenized milk is an example of a typical oil-in-water (o/w) emulsion. Milk fat (oil) is dispersed in water as fine droplets by the homogenization process. The reason the fat does not float to the top immediately is due to the presence of emulsifiers; in this case, a milk protein called sodium caseinate as well as several phopholipids. In the case of water-in-oil (w/o) emulsions, water is dispersed as droplets and suspended in the oil phase. The nondispersed liquid or external suspending phase is also called the continuous phase. Mayonnaise, vinegar water dispersed as fine droplets in a continuous phase of soybean oil, is an example of a water-in-oil emulsion. Lecithin from eggs stabilizes the mayonnaise emulsion.
Surfactants
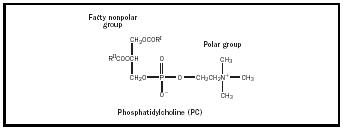
Most emulsifiers can be considered surfactants or surface-active agents. These materials are able to reduce the surface tension of water. What makes an emulsifier surface active is related to its HLB, or hydrophile-lipophile balance. HLB is determined by the size of the hydrophilic (water-loving or polar) portion of a molecule as compared to the size of the lipophilic (oilloving or nonpolar) portion. The HLB system was created to rank the relative polarity of materials. The most polar, water soluble, materials are at the top of the twenty-point scale with more non-polar, oil soluble, materials closer to zero. The HLB of sodium caseinate is assigned a value of around fourteen because of it’s high solubility in water. Lecithin, being poorly soluble in water, has an HLB value of about six. Both have polar groups. The polar group in the milk protein is sodium. Lecithin’s surface-active component is a molecule called phosphotidylcholine or PC (See Figure 1). The polar, or water soluble part of PC is the phosphate functional group. The emulsifiers’ polar groups orient toward the polar water phase. Their lipophilic, nonpolar groups oriented toward the oil phase to form micelles (see Figure 2). These spherical structures provide stability to the emulsion through Hydrogen bonding and weak electrical forces.
Skin-care emulsifiers can be divided into two groups based on ionic charge (See Figure 3). Materials that can dissociate into charged species are considered ionic while those that do not are called nonionic. Ionic emulsifiers can be further classified by type of charge. Anionics are negatively charged when solvated as in sodium stearate or soap.
When fatty acids are reacted with alkali they form soaps. The process of soap formation is called saponification. The negatively charged stearic acid group is the main emulsifying unit of the soap, giving it the anionic classification. Positively charged emulsifiers are called cationic. Quarternium24’s emulsifying unit dissociates into the positively charged ammonium group. Amphoterics are compounds that express both negative and positive charges. Read more
